![]() Posted by Marvin Calderon ⎜ Feb 7, 2020 ⎜ Industry
Posted by Marvin Calderon ⎜ Feb 7, 2020 ⎜ Industry
Abstract :
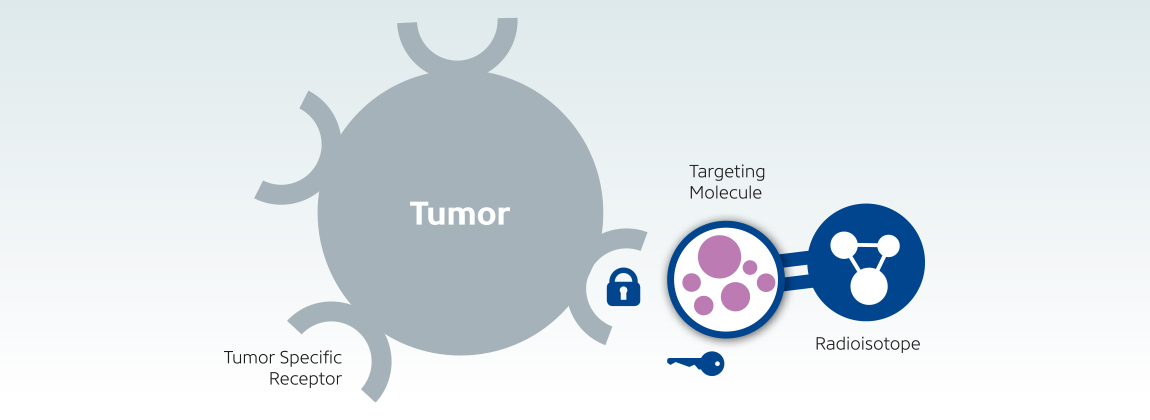
Targeted radiotherapy is an evolving and promising modality of cancer treatment. The killing of cancer cells is achieved with the use of biological vectors and appropriate radionuclides. Among the many advantages of this approach are its selectiveness in delivering the radiation to the target, relatively less severe and infrequent side effects, and the possibility of assessing the uptake by the tumor prior to the therapy. Several different radiopharmaceuticals are currently being used by various administration routes and targeting mechanisms. This article aims to briefly review the current status of targeted radiotherapy as well as to outline the advantages and disadvantages of radionuclides used for this purpose.
Introduction:
The development and evolution of modern chemotherapy during the second half of the twentieth century has improved the clinical outcome of patients with various forms of cancer. Still, in the vast majority of malignancies, the efficacy of systemic chemotherapy is very limited, with 90% of all drug cures occurring in only 10% of cancer types. Unlike systemic chemotherapy, the goal of targeted radiotherapy is the selective delivery of radiation to cancer cells in a way that causes minimal toxicity to surrounding normal tissues. The basis for successful radionuclide therapy is selective concentration and prolonged retention of the radiopharmaceutical within the tumor. Tumor response depends on numerous factors, including cumulative radiation dose delivered, dose penetration, and tumor radiosensitivity. The radiation effect is maximally achieved when the radiation dose is entirely absorbed by the tumor. In practice however, this is almost never the case because of biologic turnover.
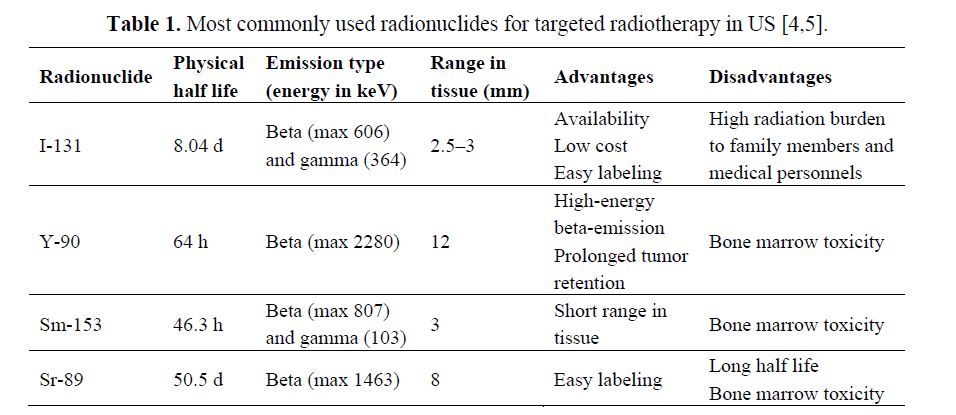
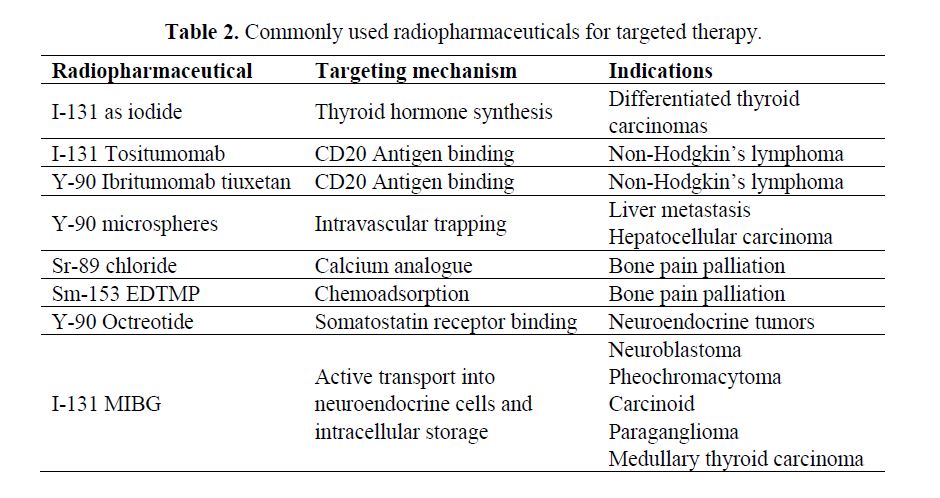
Radiopharmaceuticals and Targeting Mechanisms: The selection of the appropriate radionuclide depends on its nuclear decay properties, specifically, emission characteristics and physical half-life. The treatment of bulky tumors by radionuclides that emit high energy alpha or beta particles is the preferred approach (Tables 1 and 2); however, for the eradication of small clusters of cancer cells or small tumor deposits, radionuclides that emit Auger electrons are considered to be beneficial because of their high level of cytotoxicity and short-rangebiological effectiveness.
Conclusions:
In this article we have presented a brief review of commonly used targeted radionuclide therapeutic agents. We believe that changes in targeted radiotherapy will continue to improve alongside of progress in tumor biology research. Many institutions are formulating sophisticated radiopharmaceuticals including antibodies, fusion proteins, peptides and small organic molecules as well as sodium iodide gene transfer into tumor cells for diagnostic and therapeutic purposes.In the end, the rules in radiobiology should be exploited, in particular, radiosensitivity during G2 andmitotic phases of the cell cycle. Hence, a reasonable approach would be to select a radiolabeled agent with an effective half-life comparable to the doubling time of the tumor cells, which may be estimated from a cell culture derived from a biopsy or less precisely from imaging. Unfortunately, this approach is not practical in using long-lived isotopes due to the dosimetry of radiation burden. An alternative approach is to do serial treatments sronized to the tumor’s native biological clock or with anadjuvant cell cycle arresting agent. Randomized studies are needed to evaluate the effectiveness and practicality of this approach.
DeVita, V.T., Jr. The influence of information on drug resistance on protocol design: The Harry Kaplan Memorial Lecture given at the Fourth International Conference on Malignant Lymphoma,June 6–9, 1990, Lugano, Switzerland. Ann. Oncol. 1991,2, 93-106.
Volkert, W.A.; Goeckeler, W.F.; Ehrhardt, G.J.; Ketring, A.R. Therapeutic radionuclides: Production and decay property considerations. J. Nucl. Med. 1991, 32, 174-185.