David T. Drummond ⎜Oct 14, 2019 ⎜ Industry

BARCELONA, Spain, 13th October 2019 – McMaster University’s Nuclear Reactor (CAN) and NRG (NL) announced today a unique collaboration between the Netherlands and Canada in the field of nuclear medicine.
Working together, these two globally renowned research reactors will provide the world with the highest quality radioiodine (I-125), used for the treatment of prostate and other various types of cancers, at the highest level of reliability.
The importance of readily available medical isotopes
Tags: I-125 isoSolutions Radiochemicals
Kevin Yang ⎜Feb 13, 2019 ⎜ Industry
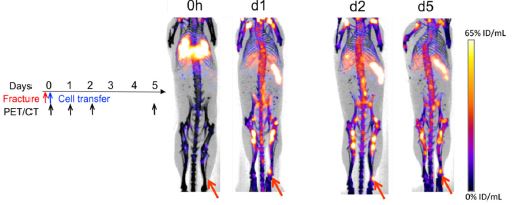
Bone fracture healing is dependent upon the rapid migration and engraftment of bone marrow (BM) progenitor and stem cells to the site of injury. Stromal cell-derived factor-1 plays a crucial role in recruiting BM cells expressing its receptor CXCR4. Recently, a CXCR4 antagonist, plerixafor, has been used to mobilize BM cells into the blood in efforts to enhance cell migration to sites of injury presumably improving healing. (more…)
Tags: Cell Tracking isoSolutions PET Radiochemicals Research Zr-89
Kevin Yang ⎜Nov 5, 2018 ⎜ Industry
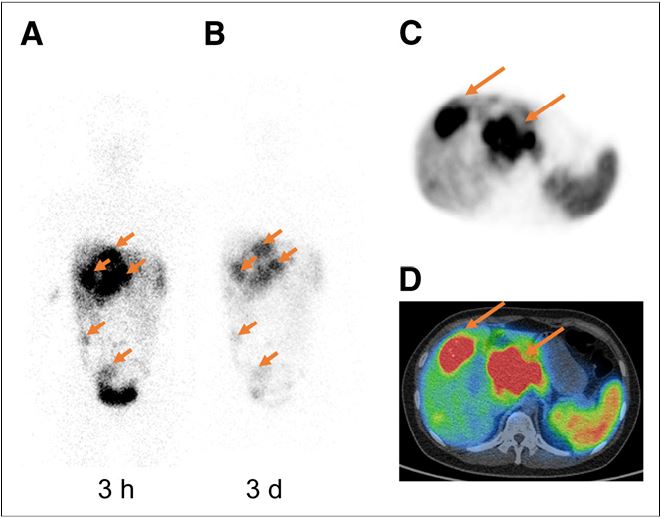
Radiolabeled somatostatin analog therapy has become an established treatment method for patients with well to moderately differentiated unresectable or metastatic neuroendocrine tumors (NETs). The most frequently used somatostatin analogs in clinical practice are octreotide and octreotate. (more…)
Kevin Yang ⎜Sep 12, 2018 ⎜ Industry
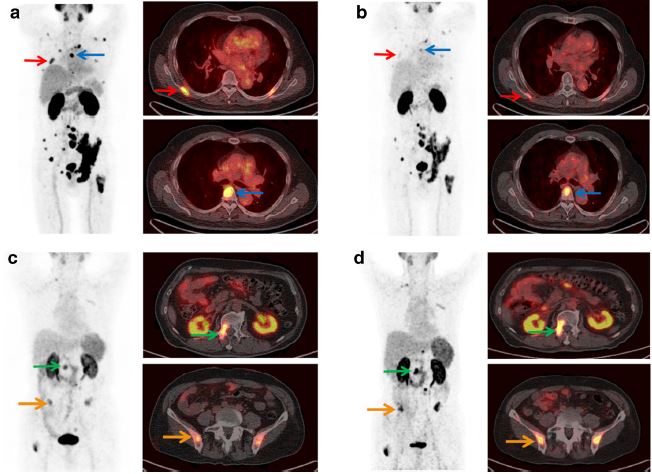
This first-in-human study demonstrated that 177Lu-EB-PSMA-617 had higher accumulation in mCRPC and that low imaging dose appears to be effective in treating tumors with high 68Ga-PSMA-617 uptakes. Elevated uptakes of 177Lu-EBPSMA-617 in kidneys and red bone marrow were well tolerated at the administered low dose. Further investigations with increased dose and frequency of administration are warranted. (more…)
Kevin Yang ⎜Sep 6, 2018 ⎜ Industry
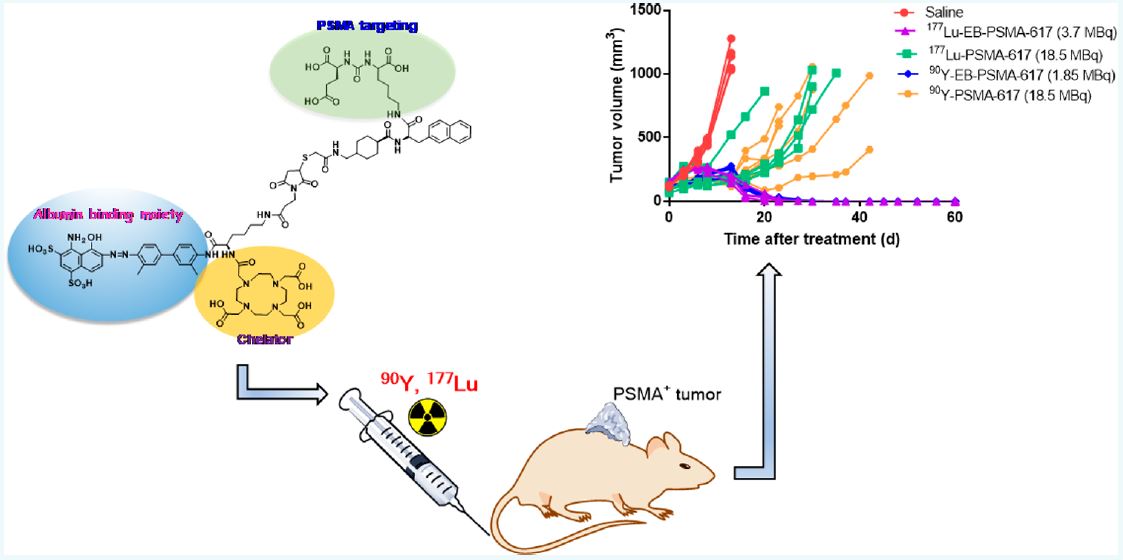
Prostate cancer is the most frequently diagnosed malignant tumor in men worldwide. Prostate-specific membrane antigen (PSMA) is a surface molecule specifically expressed by prostate tumors and has been shown to be a valid target for internal radionuclide therapy in both preclinical and clinical settings. The most common radiotherapeutic agent is the small molecule 177Lu-PSMA-617, (more…)
Hans Peng ⎜Aug 2, 2018 ⎜ Industry

Since China was the highlighted country of 2018 SNMMI in Philadelphia, several CSNM board members presented a brief report of 2018 SNMMI to the audience. One interesting fact needs attention is that Lu-177 is the most discussed topic this year not only because Lu-177 is highly in demand during SNMMI, but also more and more professors and doctors in China have realized how important and effective Lu-177 is when it is used to diagnose and treat prostate cancer. (more…)
Tags: China CSNM Lu-177 Radiochemicals SNMMI
Kevin Yang ⎜Jul 26, 2018 ⎜ Industry
Y-90 radioembolization for HCC patients with PVT appears to have an acceptable safety profile, with better survival in CP-A patients than in CP-B patients. This study confirms prior reports of survival in PVT patients treated with Y-90 radioembolization, and survival after Y-90 radioembolization appears to exceed that in
similar patients treated with systemic therapies. Despite the negative studies recently reported, Y-90 radioembolization is a reasonable treatment option in properly selected PVT patients. Further controlled studies are needed to compare it with systemic therapies or other locoregional treatments for advanced-stage HCC.
Nadine Abouchaleh1, Ahmed Gabr1, Rehan Ali1, Ali Al Asadi1, Ronald A. Mora1, Joseph Ralph Kallini1, Samdeep Mouli1, Ahsun Riaz1, Robert J Lewandowski1, and Riad Salem1–3
Info on purchasing Y-90, please contact sales@isosolutions.com
Read the full article PDF
Tags: isoSolutions Radiochemicals Y-90
David T. Drummond ⎜Jun 29, 2018 ⎜ Industry
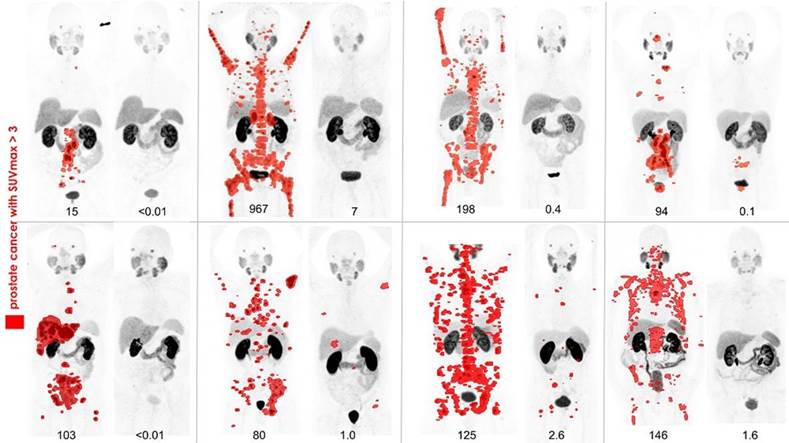
PHILADELPHIA (Embargoed until 6:30 p.m. EDT, Tuesday, June 26, 2018) ‚Äì In the battle against metastatic castrate-resistant prostate cancer, studies have demonstrated a high response rate to radionuclide therapy targeting prostate specific membrane antigen (PSMA) with the radionuclide lutetium-177 (177Lu). (more…)
David T. Drummond ⎜Jun 11, 2018 ⎜ Industry
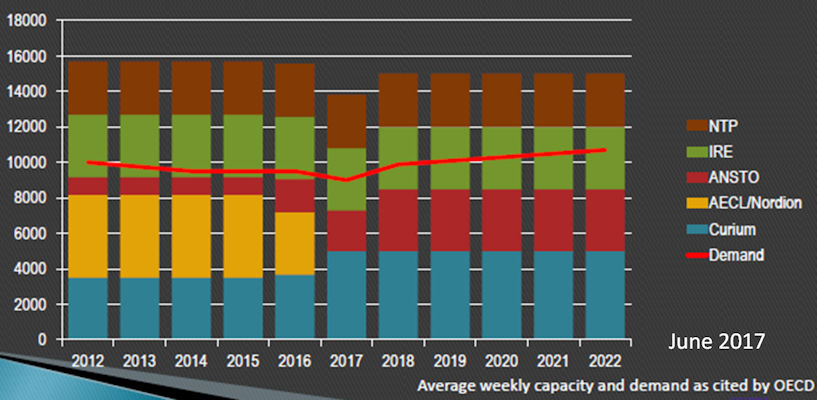
By Wayne Forrest, AuntMinnie.com staff writer.
Major changes are taking place in the intricate network that supplies healthcare providers around the world with molybdenum-99 (Mo-99), a key radioisotope for nuclear medicine studies. The question is, can the nuclear medicine community avoid another devastating shutdown like the one that occurred in 2009? (more…)
Tags: Latin America Mo-99 North America Radiochemicals Tc-99m
Kevin Yang ⎜Jun 6, 2018 ⎜ Industry
A positive response for surrogate parameters demonstrates remarkable antitumor activity for 225Ac-PSMA-617. Swimmer-plot analysis indicates a promising duration of tumor control, especially considering the unfavorable prognostic profile of the selected advanced-stage patients. Xerostomia was the main reason patients discontinued therapy or refused additional administrations and was in the same dimension as nonresponse; this finding indicates that further modifications of the treatment regimen with regard to side effects might be necessary to further enhance the therapeutic range.
Clemens Kratochwil1, Frank Bruchertseifer2, Hendrik Rathke1, Markus Hohenfellner3, Frederik L. Giesel1, Uwe Haberkorn*1,4, and Alfred Morgenstern*2
Info on purchasing Ac-225, please contact sales@isosolutions.com .
Tags: isoSolutions Radiochemicals
David T. Drummond ⎜Mar 14, 2018 ⎜ Industry
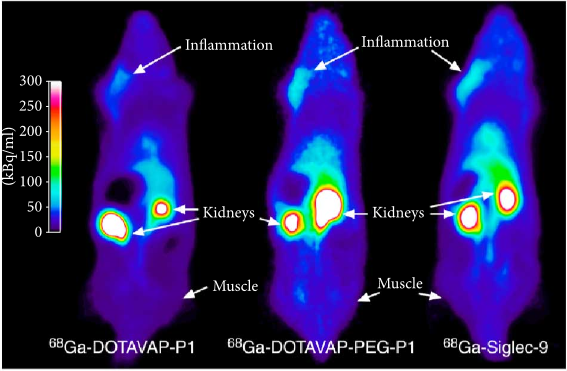
During the last decade, the utilization of 68Ga for the development of imaging agents has increased considerably with the leading position in the oncology. The imaging of infection and inflammation is lagging despite strong unmet medical needs. This review presents the potential routes for the development of 68Ga-based agents for the imaging and quantification of infection and inflammation in various diseases and connection of the diagnosis to the treatment for the individualized patient management. (more…)
Tags: Ga-68 PET Radiochemicals
David T. Drummond ⎜Feb 1, 2018 ⎜ Products

IRE is proud to announce a partnership with ASML to further develop a new, non-fission production method for medical isotopes such as Mo-99/Tc-99m. This partnership has resulted in the start of the LightHouse Isotopes BV, an ambitious initiative for the development of a promising alternative production method for a sustainable long term supply of Mo-99.
Click here to see full post.
Tags: Mo-99 Radiochemicals Tc-99m